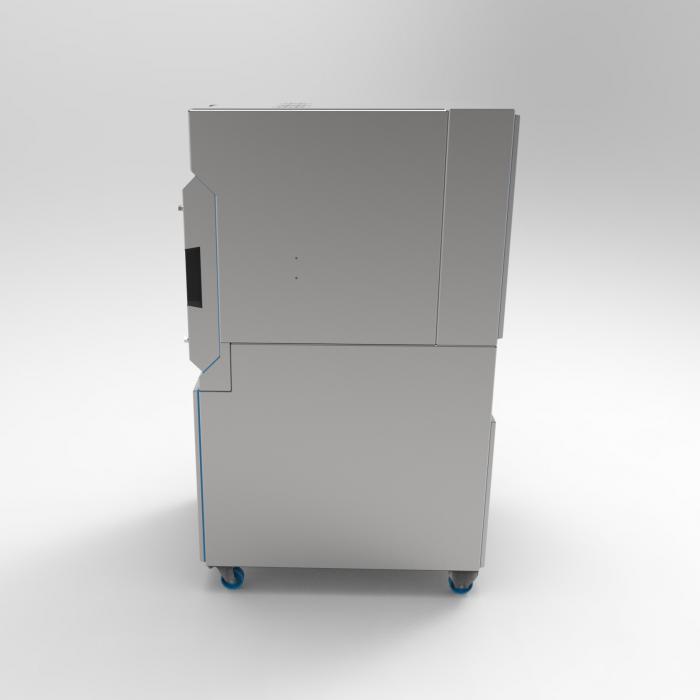
ECH-One The first dust collector truly designed for tabletting, capsule filling and primary packaging.


ECH-One Mobile Dust Collector
ECH-One is a safe and compliant companion for your tablet presses, capsule fillers, and primary packaging machines. The ECH-One is engineered to eliminate the significant GMP, safety, and product loss risks associated with using repurposed industrial vacuum cleaners in sensitive pharmaceutical applications.
Compact & Mobile
Delivers a powerful 900 m³/h (530 cfm) of airflow from a minimal 0.8 m² (9 ft²) footprint, mounted on wheels for easy mobility.
GMP-Compliant by Design
Features a cleanable design with FDA-compliant materials, individually leak-tested housing (ISO 10648-2), and HEPA filter integrity test ports to meet stringent GMP regulatory requirements.
Uncompromised Safety
Offers optional OEB 5 containment for handling highly potent APIs (OEL>0.9 microg/m³) and is designed for explosion protection in compliance with ATEX and NFPA 654 standards.
Plug & Play Simplicity
Fully electric system with an integrated fan and intuitive HMI, ready for operation in under 5 minutes without the need for compressed air.
Modular & Configurable
Available in three distinct lines (BaseLine, SafeLine, PureLine) with a wide range of options to perfectly match your application's specific containment and compliance needs.
Are You Aware of the Risks in Your Process?
Pharmaceutical manufacturers face substantial risks when using standard industrial vacuum cleaners as dust collectors. These can lead to FDA warning letters, multi-million dollar fines, production losses, and damage to your company's reputation. The ECH-One was engineered from the ground up to address these critical challenges directly.
Solving the Core Challenges of Pharmaceutical Dust Extraction
Eliminate GMP & Cross-Contamination Risks
Industrial vacuum cleaners present serious compliance gaps. Their HEPA filters often cannot be tested in-situ, and housing tightness is not confirmed. This has been cited in FDA Warning Letters in the past, so pharmaceutical companies should protect for the future. The ECH-One provides a:
Validation & Liability Shield with:
- A cleanable design using GMP & FDA compliant materials.
- Individually leak-tested housing as per ISO 10648-2.
- Triple-stage filtration with double HEPA safety filters.
- Built-in filter integrity test ports (ISO 16170) for routine validation.
Ensure Uncompromised Personnel Safety
Standard industrial vacuum cleaner systems create a false sense of safety, with undetected leaks in gaskets and housings potentially exposing operators to hazardous APIs. The ECH-One is built for:
Uncompromised Safety with:
-
Available high-containment solutions for OEB3, OEB 4 and OEB 5 applications.
-
Safe-change filter and dust bin procedures to minimize exposure risk.
- Filter doors used as a tray, for a safer and more ergonomic filter change.
-
Explosion protection for combustible dusts (MIE > 3 mJ) compliant with ATEX and NFPA standards.
Protect Your Product Yield & Formulation
High-pressure blowers without proper regulation can cause excessive loss of expensive product to the exhaust. Worse, this can lead to the selective loss of specific ingredients, impacting the final formulation's integrity. The ECH-One ensures
Stable Process Conditions through:
- An integrated fan with VFD and PID control for stable pressure conditions.
- An optional constant airflow regulation system for the most stable production conditions.
- Triple-stage filtration including a primary cleanable filter with ROTATRONIC-L online cleaning technology to maintain performance.
Dust Collector Specifications
General Specifications
Features |
Details |
| Size | 159 x 99 x 78 cm (62 x 39 x 31") |
| Mobility | On castor wheels |
| Air flow capacity | 100−900 m³/h (60 - 530 cfm) |
| Fan static pressure | 10 kPa @ max air flow (40 in. WC) |
| Fan control | VFD control with PID (dP) regulation |
| Air exhaust | Into room or outside via ductwork |
| Number of filter stages | 2 (E12+H14) |
| Explosion protection | Combustible dusts, MIE > 3 mJ, ATEX and NFPA compliant |
| Control system | Siemens, with 4" HMI |
| Compliance | CE or UL |
|
Documentation pack |
|
Modular by Design: The Right ECH-One for Your Application
The ECH-One is available in three standard configurations to provide a tailored solution for your specific operational needs.
Features |
BaseLine |
SafeLine |
PureLine |
|
Housing material |
Powder coated steel |
Powder coated steel |
Stainless steel, Ra < 1.6 |
| Containment | None |
BiBo (OEB3) |
BiBo (OEB3) |
|
Dust extraction |
30 L PE bag |
Safe-change 30 L bag |
Safe-change 30 L bag |
|
Equipment connectivity |
Hardwired - basic |
Hardwired - extended |
Hardwired - extended |
| Filter integrity testing | ✗ | ✓ | ✓ |
|
GMP/FDA compliance |
✗ | ✗ | ✓ |
|
Advanced options possible |
✗ | ✓ | ✓ |
|
Doc. and service options |
✓ | ✓ | ✓ |
Advanced Options & Connectivity
Further enhance your ECH-One with powerful options for ultimate safety, performance, and integration.
High Containment (OEB4 & OEB5)
For uncompromised safety with toxic APIs, this option includes an individually leak-tested housing and bags (ISO 10648-2, Class 3), a special tightness safe-change bag, a 15 L continuous liner, and a filter tightness tray for increased containment during filter change.
Additional Safety Filtration
For maximum air return reliability, this adds a third H14 filter stage, filter-in-place detection for all three filter stages, and filter sealing test ports on all filters.
Constant Air Flow Regulation
Ensures the most stable production conditions with integrated air flow measuring equipment, sensors, and additional software functions.
Isolation with Valve
Includes integrated isolation valve with electrical drive and the associated SW controls, ensuring unit isolation during a standstill.
Seamless Integration
Equipped with Siemens control components and a proprietary ROTATRONIC HMI. Go beyond hardwired I/O with full PROFINET or OPC UA connectivity for complete communication with your process equipment or BMS.
Documentation & Service Options
For full compliance and peace of mind, consider the following documentation and service options.
GMP Documentation
GMP documentation pack includes Installation Qualification (IQ) and Operational Qualification (OQ) as well as Software Design Specification (SDS), Hardware Design Specification (HDS) and Functional Specification (FS) documents.
Factory Acceptance Test (FAT)
FAT option includes FAT documentation preparation and on-site or online FAT execution by TRM team.
Hard Copy Documentation
The option includes entire documentation pack in paper format, in addition to e-documentation which is included as a standard.
Documentation & Labelling Language
Besides standard documentation languages (free of charge) - Slovenian, English, French, German, Spanish, Swedish, Dutch - the package includes translation of documentation and labelling to any world language.
Protect Your People, Product, and Process Today.
Stop accepting the risks of inadequate dust filtration. The ECH-One is the purpose-built solution to ensure safety, compliance, and efficiency in your pharmaceutical manufacturing suite.
Contact our filtration experts to discuss your application, configure your ideal ECH-One system, and receive a personalized quote.